NEW WEBSITE: SUBLIMINAL LASER THERAPY FOR RETINAL DISORDERS

Laser therapy has been used to treat retinal pathologies for decades. Over the years, the development and introduction of new and innovative laser technologies has confirmed that, alongside intravitreal injections, retinal laser therapy still plays a valuable role in the surgeons’ treatment armamentarium.
Lumibird Medical’s glaucoma treatment potential further strengthened by the latest EGS recommendations

The European Glaucoma Society (EGS), the world’s foremost learned society for glaucoma and its treatments, issued recommendations in its fifth “Terminology and Guidelines for Glaucoma” report, published in March 2021, that validate the positioning and relevance of the solutions developed by LUMIBIRD Medical. EGS officially added SLT…
Creation of 3 Lumibird Medical Nordics subsidiaries

The Lumibird Medical Group, the medical subsidiary of the Lumibird Group, the European leader in laser technologies, today announces the creation of Lumibird Medical Nordics AB in Sweden, Lumibird Medical Nordics OY in Finland and Lumibird Medical Nordics AS in Norway.
MOSAR® FOR EASYRET®: HIGH DEFINITION IMAGING SYSTEM
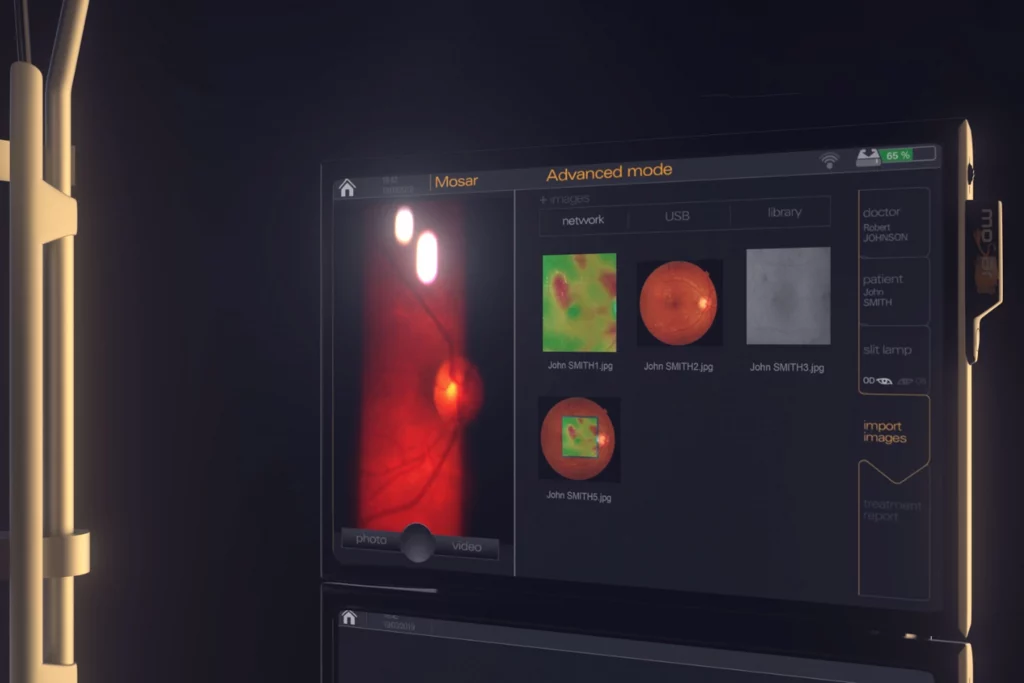
Quantel Medical accessorizes its yellow laser Easyret® with Mosar®, a High definition imaging system.
Always tuned to the market needs and to the feedbacks of the ophthalmologists, Quantel Medical launches Mosar®, a new optional imaging system dedicated to its high-end yellow retinal laser.
QUANTEL MEDICAL COMPLETES ACQUISITION OF ELLEX

Quantel Medical has completed its acquisition of Ellex, including the company’s laser and ultrasound technology solutions (with the exception of 2RT® and iTrack®), via the financial holding company Lumibird Medical.
Quantel Medical and Ellex announce merger agreement

Quantel Medical announces a major step forward for Quantel Medical and the Lumibird group as merger discussions have been initiated with Ellex, Adelaide, Australia.
VITRA 2 RECEIVES APPROVAL FROM THE FDA

Quantel Medical announced today that it has received approval from the U.S. Food and Drug Administration (FDA) for the Vitra 2® photocoagulator.
Acquisition of the company Optotek Medical

Quantel Medical announces the acquisition of the Slovenian company Optotek Medical d.o.o., specialised in the development of optical solutions and lasers for medical applications.
LacryStim: CE mark approval
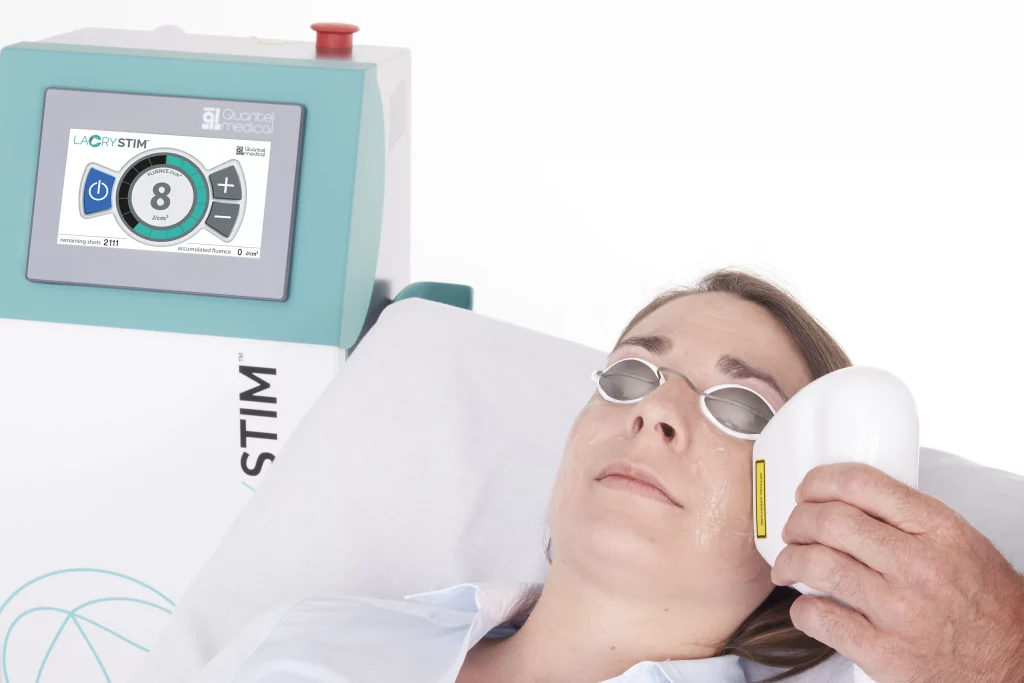
Dry eye disease is a multifactorial disease of the tears and ocular surface that can result in ocular discomfort and visual impairment. It affects all populations across the world with a major increase in people over 50 years old and is expected to increase as the population continues to age.
Quantel Medical receives FDA approval for the new A/B/S/UBM ultrasound platform ABSolu®
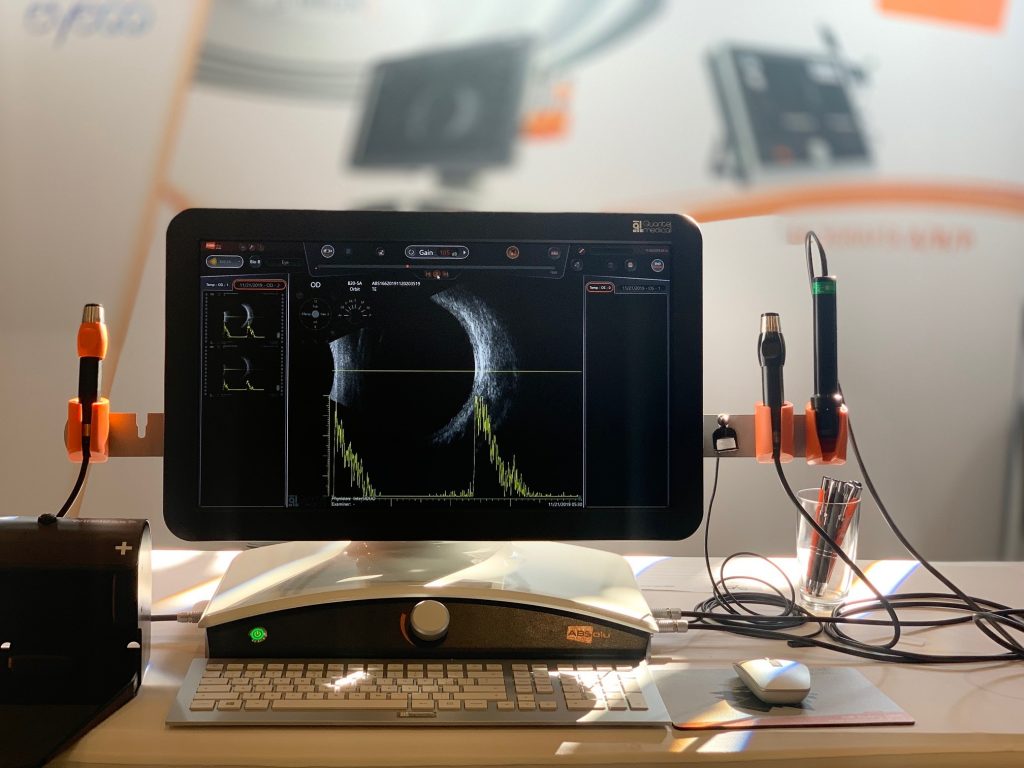
Quantel Medical today announced that it has received approval from the U.S. Food and Drug Administration (FDA) on March 25, 2019 for the New A/B/S Ultrasound Platform: ABSolu® .